
Click on each organ to know more about cGvHD
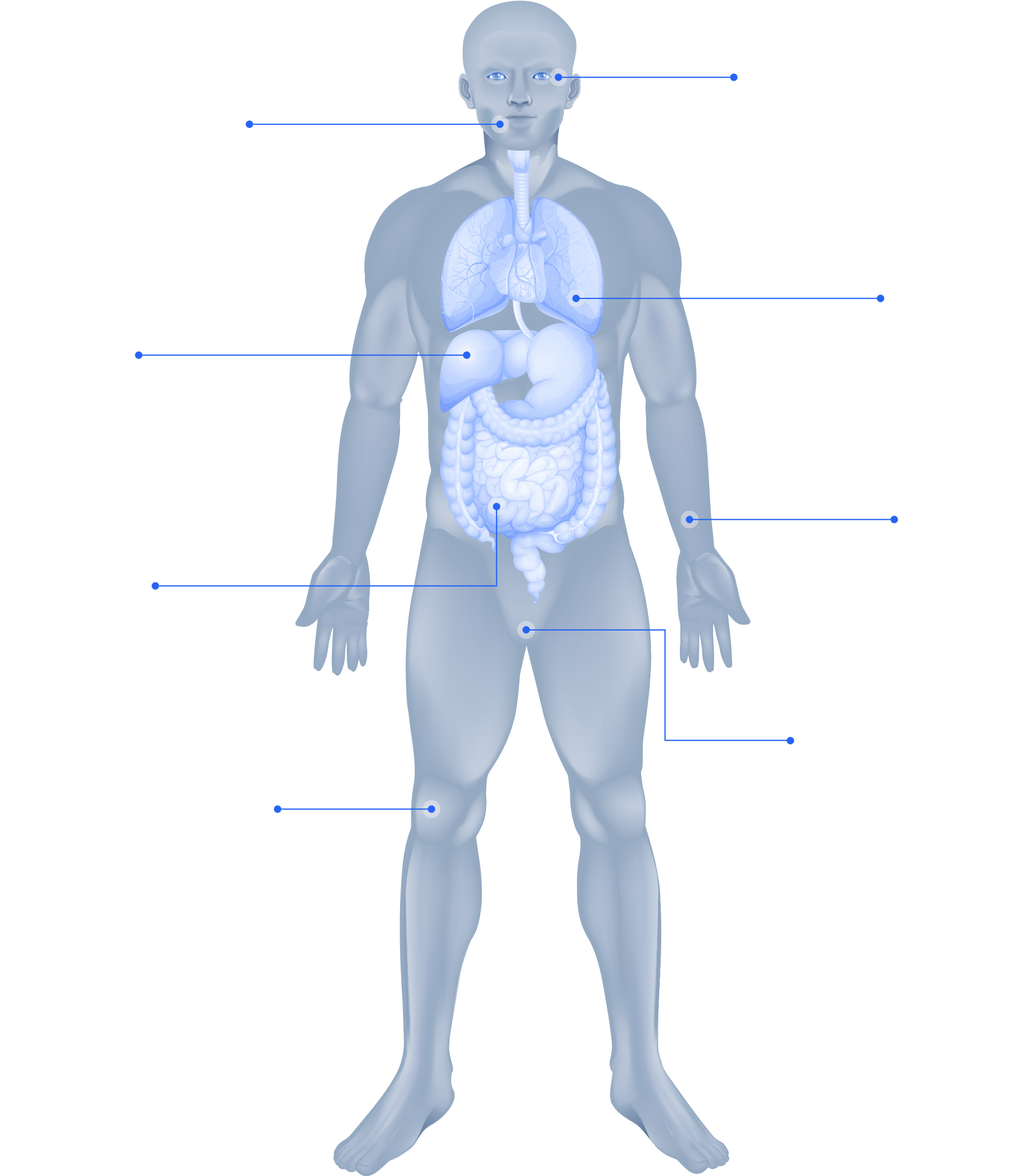
Eyes
Mouth
Lungs
Liver
Skin
GI tract
Genitalia
Joints


Eyes
Mouth
Lungs
Liver
Skin
GI tract
Genitalia
Joints
GvHD is a common systemic complication that can occur post alloHCT1-3

GvHD is characterized into aGvHD or cGvHD based on clinical features1

aGvHD vs cGvHD

AlloHSCT recipients can develop one, both or neither type of GvHD.4,5
Thymus destruction and deficient selection of donor T cells by the thymus are major factors contributing to
allo- and autoimmunity associated with cGvHD.8
Early damage to the B cell niche in the bone marrow disturbs B cell development, leading to elevated BAFF levels, which predict cGvHD, and insufficient elimination of B cells producing auto- and allo antibodies.8
A hallmark of cGvHD is the development of sclerotic lesions, which can occur in almost every organ.8
Reactive T-cells and B-cells promote monocytes to differentiate into fibroblast activating tissue-resident macrophages, leading to fibrosis.9
The conditioning regimen causes the destruction of epithelial cells and compromises their integrity.
The damaged epithelial cells release uric acid and ATP, which in turn leads to the production of pro-inflammatory cytokines.
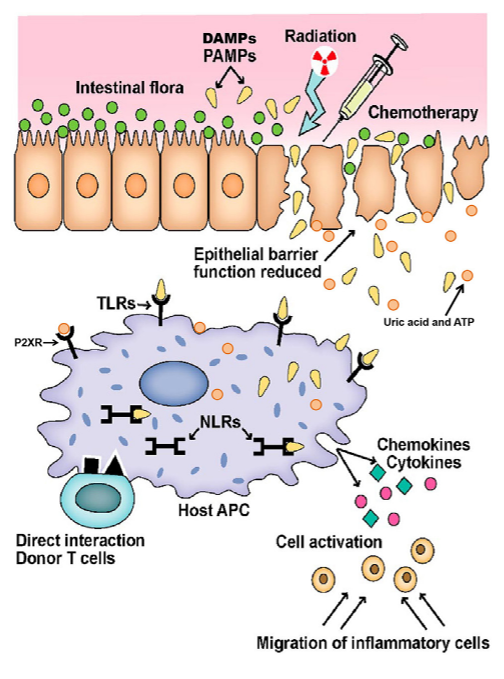
This image was obtained from an open-access article distributed under the terms of the Creative Commons Attribution License (CC-BY)
aGvHD, acute graft-versus-host disease; Allo, allogenic; alloHSCT, allogeneic hematopoietic stem cell transplant; BAFF levels, B cell activating factor; B cells, bursa-derived cells; cGvHD, chronic graft-versus-host disease; T cells, T lymphocytes; PNS, peripheral nervous system; MSK, musculoskeletal system; GI, gastrointestinal; GU, genitourinary; AIHA , autoimmune hemolytic anemia; AIN, autoimmune neutropenia; PPFE, pleuroparenchymal pulmonary fibroelastosis; COP, cryptogenic organizing pneumonia.
1. Justiz Vaillant AA et al. In: StatPearls. StatPearls Publishing; 2020. Updated May 1, 2022. Accessed July 18, 2023. https://www.ncbi.nlm.nih.gov/books/NBK538235; 2. Henden AS, Hill GR. J Immunol. 2015;194(10):4604-4612. doi:10.4049/jimmunol.1500117; 3. Ramachandran V et al. Dermatol Clin. 2019;37(4):569-582. doi:10.1016/j.det.2019.05.014; 4. Lee SJ. Blood. 2017;129(1):30-37. doi:10.1182/blood-2016-07-686642; 5. Jagasia MH et al. Biol Blood Marrow Transplant. 2015;21(3):389-401.e1. doi:10.1016/j.bbmt.2014.12.001; 6. Ferrara JLM et al. Lancet. 2009;373(9674):1550-1561. doi:10.1016/S0140-6736(09)60237-3; 7. Mankarious M et al. Front Immunol. 2020;11:81. doi:10.3389/fimmu.2020.00081; 8. Ghimire S et al. Front Immunol. 2017. 10.3389/fimmu.2017.00079.; 9. Kelli P.A Macdonald et al. Blood. 2017;129(1):13-21; 10. Zeiser R, Blazar BR. N Engl J Med. 2017;377(26):2565-2579. doi:10.1056/NEJMra1703472; 11. MacDonald KPA et al. Blood. 2017;129(1):13-21. doi:10.1182/blood-2016-06-686618; 12. Kitko CL et al. Biol Blood Marrow Transplant.2012;18(suppl 1):S46-S52. doi:10.1016/j.bbmt.2011.10.021; 13. Cooke KR et al. Biol Blood Marrow Transplant. 2017;23(2):211-234. doi:10.1016/j.bbmt.2016.09.023. 14. Cuvelier GDE et al. Transplant Cell Ther.2022;28(8):426-445.